Transition™
Uncemented Hip Replacement System
Transition™ design is based on a clinically proven1,2,3,4,5,6 femoral stem design which was developed in France during the early 1980's by the ARTRO Group, who trained together in Lyon.
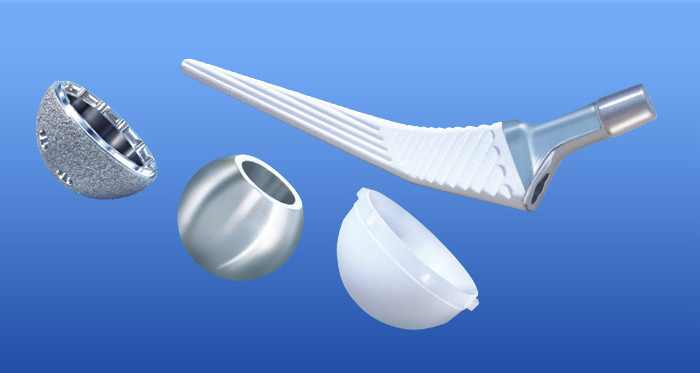
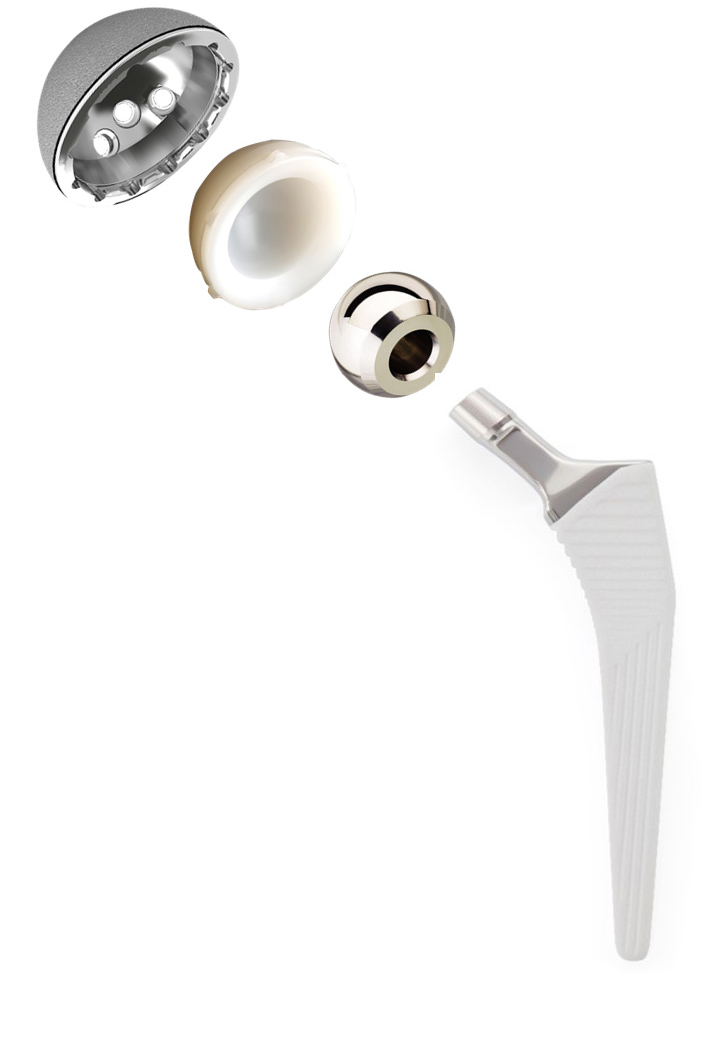
Transition™ is a collarless, Cementless, fully coated hydroxyapatite(HA) implant available in a broad range of sizes, allowing surgeons to choose the right solution for Individual patient needs.
High performance instrumentation facilitates the Surgeon for a simple and accurate implantation with reproducible results.
Transition Hip Product Manual-cdr-compressed

Altius Cemented Hip Product Manual-compressed
Altius Bipolar Hip Product Manual
Key Benefits
- Proven design, Material and Fixation - cementless tapered rectangular design, titanium, Hydroxyapatite (HA) coated.
- Ergonomic and efficient design and layout.
- Suitable for different anatomies and varying bone quality.
- Biocompatible, Osteoconductive surface for an enhanced bone integration.
- Polished Neck design offers an improved ROM for the patient with reduced opportunities for impingement
Osprovit® Technology
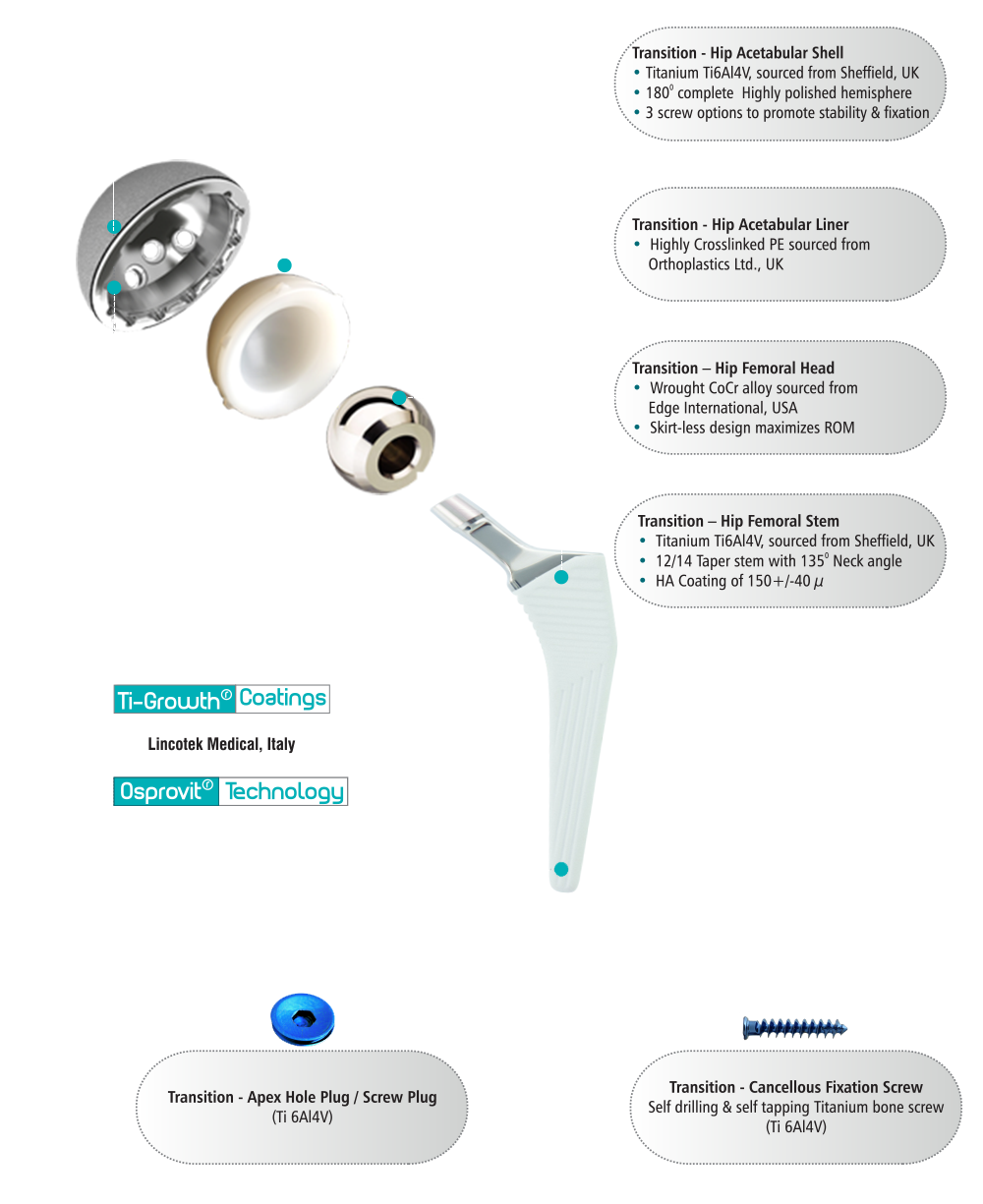
Transition™ stem using Osprovit®- Patented Plasma Spray Hydroxyapatite (HA) coatings from Lincotek Medical S.p.A, Trento, Italy (Formerly Eurocoating)
Osprovit® is a slowly bioresorbable, bioactive HA coating that has a long clinical history, that guarantees its high performance and suitability for widespread application.
Osprovit® is typically produced as the top HA layer when a double layer coating is done under vacuum. This application combines the bioactive performance of HA with the long-term mechanical stability of porous titanium.
A considerable amount of biological and clinical evidence is available to confirm the optimal biocompatibility and long-term clinical safety and efficacy of Osprovit®.
Ti-Growth® Coatings
Transition™ Acetabular cups are Titanium Plasma Spray( TPS) Coated, which includes Ti-Growth®, patented TPS coating from Lincotek Medical S.p.A, Trento, Italy ( Formerly Eurocoating)
Ti-Growth® provides an outstanding, unique, new solution to spray a metallic trabecular structure onto your device.
Designed to allow bone in-growth and is the first titanium (Ti) foam that sprayed onto Transition™ acetabular cups. Conventional cementless prostheses surface roughened by sandblasting, etching or plasma spray coating and allow bone apposition (on-growth) only but Ti-Growth® coatings, have specifically been designed to have large-sized pores (100–800 microns) and high porosity (30-70%) to allow bone in-growth.
Bone In-Growth Design
Ti-Growth® consists in a series of open and interconnected large size pores arranged in a Ti matrix.
The porosity profile of Ti-Growth® is not an ordered structure like porous beads, but rather a completely random structure that is rough and porous at the same time.
Advances in process control have enabled the application of thick coatings (up to 1000 microns), which allows for high and interconnecting porosity that are suitable for joint replacement components.
Sizing Chart
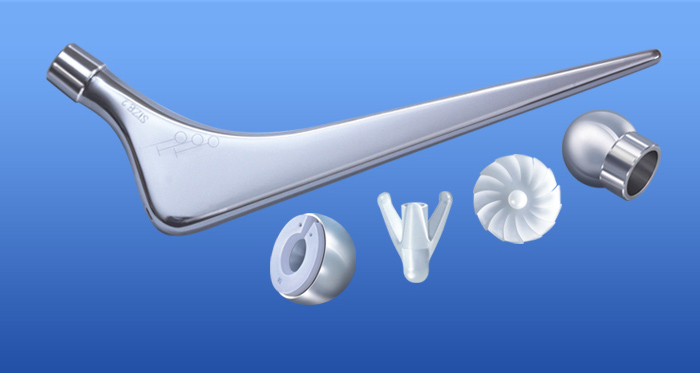
Transition - Hip Femoral Side Options
| Transition Uncemented Hip Femoral Stem Sizes | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| Stem length( mm ) | 115 | 130 | 140 | 145 | 150 | 154 | 160 |
| Offset(mm) | 38.1 | 38.7 | 38.8 | 40 | 40.8 | 41.4 | 41.8 |
| Neck Angle | 135° | ||||||
Transition - Hip Acetabular Side Options
| Hip Acetabular Shell (OD in mm) | 40 | 42 | 44 | 46 | 48 | 50 | 52 | 54 | 56 | 58 | 60 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hip Femoral Head (OD in mm) | 40 | 42 | 44 | 46 | 48 | 50 | 52 | 54 | 56 | 58 | 60 |
| Hip Acetabular Liner (OD in mm) | 22 | 22 | 22 | 28 | 28 | 28/32 | 28/32 | 32/36 | 32/36 | 32/36 | 36 |
| Hip Femoral Head- Offset (mm) | +8, +4, 0 | -4 , 0 ,+4 , +8 | |||||||||
| Transition - Cancellous Fixation Screws | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Screw Length (mm ) / 6.5 mm Dia | 20 | 25 | 30 | 35 | 40 | 45 | 50 |
Extra Resources
| Transition Product Manual |
References
- National Joint Registry for England and Wales 2011 Annual Report.
- Australian National Joint Register 2011 Annual Report.
- Epinette JA, Geesink RGT. Hydroxyapatite Coasted Hip and Knee Arthroplasty. Expansion ScientifiqueFrancaise. 1995. 2-7046-1470-9.
- Epinette JA, Manley MT. Fifteen Years of Clinical Experience with Hydroxyapatite Coatings in Joint Arthroplasty. Springer-Verlag. 2004. 2-287-00508-0.
- Geesink RGT, Manlet MT. Hydroxylapatite Coatings in Orthopaedic Surgery. Raven Press. 1995. 0-7817-0005-1.
- Hallan G, Lie SA, Furnes O, Engesaeter LB, Vollset SE, Havelin LI. Medium. and long term perfromance of 11516 uncemented femoral primary stems from the Norwegian arhtroplasty register. J Bone Joint Surg [Br] 2007 Dec;89(12): 1574-80.